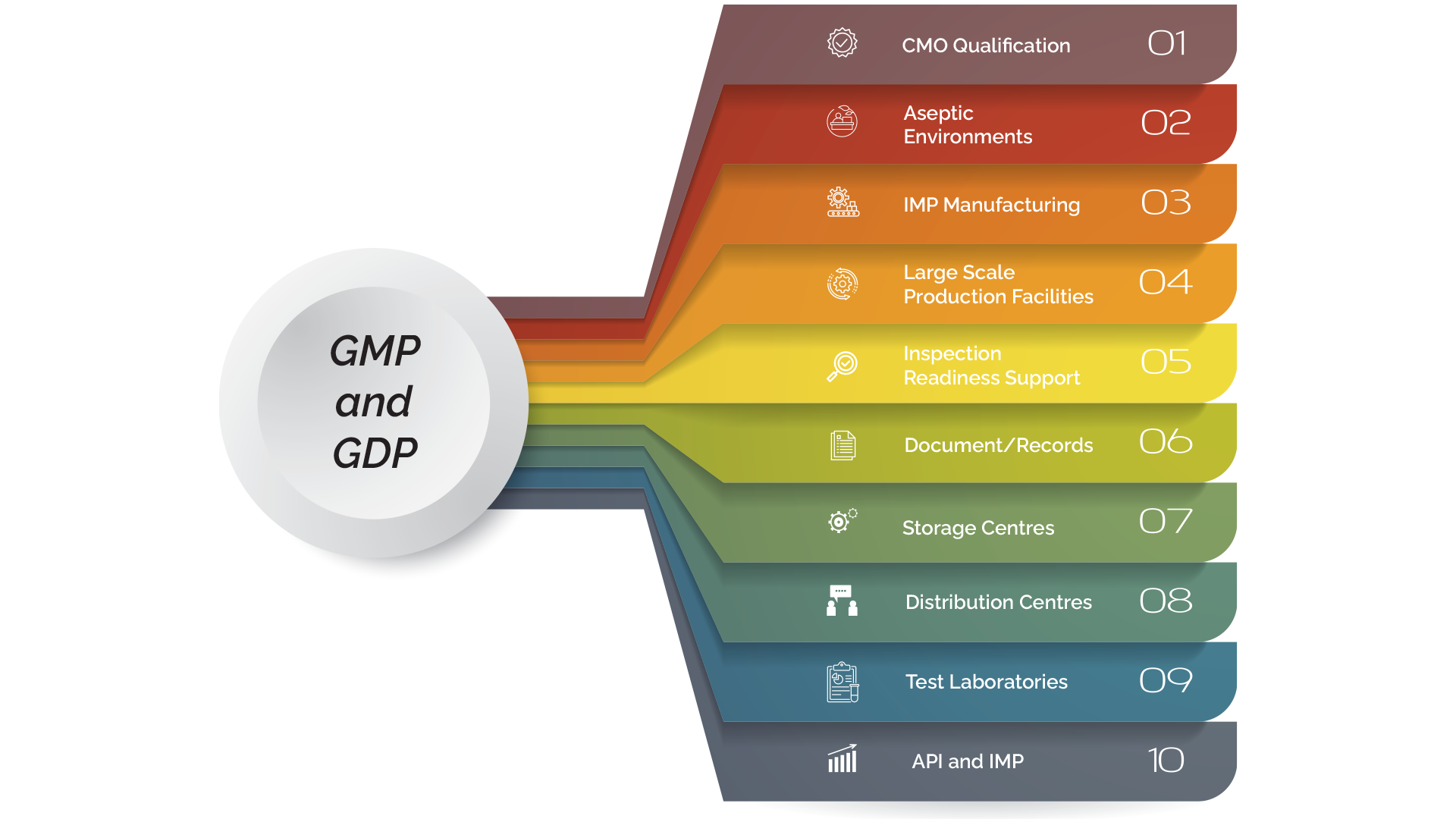
Good manufacturing practice (GMP) verifies that products are manufactured in compliance with and monitored against quality standards that are important for their intended use and comply with requirements set forth by medical authorities. economic set. With experience in GMP codes and a knowledgeable team of auditors, Mivigilance can identify and define your client's needs in accordance with GMP guidelines (compliance or certification requirements). Our GDP experts/audits are familiar with the demanding world of quality, from the initial supply of raw materials to the final manufactured drug to the end user. Thanks to our flexibility and extensive network, we provide a wide range of GMP/GDP services according to EU GMP guidelines, US cGMP regulations; ICH Q7 – GMP (Guidelines for Good Manufacturing Practices for Active Pharmaceutical Ingredients) and GMP (Good Distribution Practices in accordance with EU GMP Guidelines). Our range of GMP/GDP services include:
- CMO Qualificationl
- Aseptic Environments
- IMP Manufacturing
- Large Scale Production Facilities
- Inspection Readiness Support
- Document/Records
- Storage Centres
- Distribution Centres
- Test Laboratories
- API and IMP